YOUR CART
- No products in the cart.
Subtotal:
$0.00
BEST SELLING PRODUCTS
$110.00 – $425.00
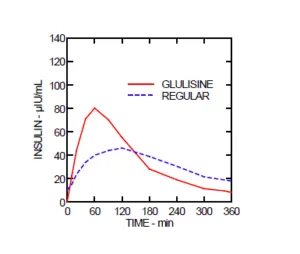
The Apidra SoloStar Pens are a rapid-acting insulin (similar to Fiasp) that begins working 10 to 20 minutes after application. Like insulin produced in the body, it allows glucose into fat cells and muscles to have energy. In addition, it will block glucose from being released from the liver.
Apidra SoloStar Pens (100IU/ml injectable) are used to improve blood sugar levels in people with type 1 and type 2 diabetes. Fast-acting insulin effectively lowers blood sugar levels after meals and helps prevent severe diabetes complications.
Apidra is also available in vials and cartridges. Solostar pens and similar cartridges are available through the Apidra brand.
Diabetes medications or other long-acting insulin medications are typically prescribed with this medication. This insulin is to be injected subcutaneously (under the skin) by your nurse or doctor. You should exercise regularly, eat a healthy diet, and lose weight as part of your diabetes treatment.
Track your blood sugar levels regularly, and be sure to share them with your doctor so they can adjust your dosage accordingly. Your doctor may add other medications to your regimen such as Novolin to help better your diabetes management.
Patients should not share SoloStar pens, even if the needle is changed. Needles and syringes must never be shared or reused by patients who use Apidra vials. Shared devices can transmit blood-borne pathogens.
Patients on insulin therapy must monitor their glucose levels. Under medical supervision, insulin regimen changes should be made cautiously. It may be necessary to change insulin doses due to changes in insulin strength, manufacturer, type, or method of administration. It may be necessary to adjust oral antidiabetic treatment concomitantly. The time course of action for Apidra may vary among individuals or at different times within the same individual depending on many factors, including the site of injection, local blood supply, and local temperature. Changing physical activity levels or meal plans may require adjustment of insulin dosages.
The most common side effect of insulin therapy, including Apidra, is hyperglycemia. Tighter glycemic control increases the risk of hypoglycemia. Patient education is essential to recognizing and managing hypoglycemia. Hypoglycemia can result in unconsciousness and/or convulsions, or may even cause death. Clinical trials with insulin, including trials with Apidra, have shown cases of severe hypoglycemia requiring another person’s assistance and/or parenteral glucose infusions and glucagon administration. Hypoglycemia is usually related to the time-action profiles of insulin formulations. Hypoglycemia may also be caused by other factors such as changes in food intake (e.g., amount of food or timing of meals), injection site, exercise, and concomitant medications]. With all insulin, use caution with patients who are unaware of hypoglycemia or who may be predisposed to hypoglycemia (e.g., children and people who fast or eat irregularly). As a result of hypoglycemia, the patient may have difficulty concentrating and responding. Especially in situations where these abilities are key, such as when driving or operating machinery, this can pose a risk. Patients with diabetes may experience symptoms similar to hypoglycemia when their serum glucose levels change rapidly, regardless of the glucose value. Hypoglycemia’s early warning symptoms may differ or diminish under certain conditions, including long-term diabetes, diabetic nerve disease, use of beta-blockers, or intensified diabetes control. Hypoglycemia in these situations may result in severe hypoglycemia (and, possibly, loss of consciousness) before the patient can recognize it. Insulin administered intravenously has a more rapid onset of action than insulin administered subcutaneously, requiring closer monitoring for hypoglycemia.
With insulin products, including Apidra, there is the possibility of life-threatening, generalized allergies, including anaphylaxis.
Apidra, like all insulin products, causes a shift in potassium between the extracellular and intracellular spaces, potentially resulting in hypokalemia. Hypokalemia untreated can cause respiratory paralysis, ventricular arrhythmia, and death. Patients who may be at risk for hypokalemia (e.g., patients taking potassium-lowering medications, patients taking medications sensitive to serum potassium levels) should be treated with caution. When Apidra is administered intravenously, monitor glucose and potassium frequently.
Patients with renal or hepatic impairment may require frequent glucose monitoring and insulin dose reduction.
Insulin preparations other than NPH insulin should not be mixed with Apidra for subcutaneous injection. When mixing Apidra with NPH insulin, Apidra should be drawn into the syringe first. Immediately follow mixing with injection. Using Apidra in a continuous subcutaneous infusion pump or intravenous administration with other insulins is not recommended. In addition to 0.9% sodium chloride (normal saline), Apidra should not be diluted with other solutions. Apidra has not been tested for efficacy and safety in external subcutaneous infusion pumps when mixed with diluents or other insulins.
Apidra must never be diluted or mixed with any other insulin when it is used in an external insulin pump for subcutaneous infusion. The reservoir of Apidra must be changed every 48 hours at the very least. Temperatures greater than 98.6°F (37°C) should not be exposed to Apidra. Hyperglycemia, ketosis, and diabetic ketoacidosis can occur rapidly when the insulin pump or infusion set malfunctions or handling errors occur. Diabetic ketoacidosis or hyperglycemia must be identified and corrected as soon as possible. Apidra is sometimes necessary for interim treatment. In addition to training to administer insulin by injection, patients using continuous subcutaneous insulin infusion pumps must have alternative insulin therapy options available.
To avoid potentially fatal hypoglycemia and hypokalemia, glucose and potassium levels must be closely monitored when Apidra is administered intravenously. Apidra should not be mixed with other insulin for intravenous administration. Only normal saline solution can be diluted with Apidra.
In patients taking thiazolidinediones (TZDs), which act as PPAR gamma agonists, fluid retention can occur, particularly in those taking insulin. Heart failure may be exacerbated or caused by fluid retention. It is important to monitor patients treated with insulin, Apidra, and PPAR-gamma agonists for signs and symptoms of heart failure. The PPAR-gamma agonist should be discontinued or reduced in dose if heart failure develops according to current guidelines.
In order to prevent disease transmission, do not share needles, cartridges, or syringes. Injectables should be used only once. Injection pens should not be shared with anyone, even if the needle has been changed. One needle should be used per individual.
Attach a new needle every time you use it. Choose a gauge and length that suits you. Make sure that the needles you use with SoloStar are approved by the manufacturer. Consult your doctor about Apidra and ensure it is right for you. Ask your doctor for more information.
You will not be able to select a dose when there is no needle attached to the injection button.
Always perform the safety test before each injection.
If you are given an injection by someone else, they must take particular care to avoid needle injuries and infection transmission.
SoloStar should never be used if it is damaged or if you are not sure it is working properly.
If you lose or damage your SoloStar, always have a spare on hand
Many medications can cause side effects. A side effect is an unwanted response to a medication when it is taken in regular doses. Side effects can be mild or severe, temporary or permanent. The side effects listed below are not experienced by everyone who takes Apidra Solostar (Insulin-glulisine-rapid). If you are concerned about side effects, discuss the risks and benefits of Apidra Solostar (Insulin-glulisine-rapid) with your doctor.
The following side effects have been reported by at least 1% of people taking Apidra Solostar (Insulin-glulisine-rapid): redness, itching, swelling, or bleeding at the place of injection, thickening of the skin at the injection site, anxiety, blurred vision, confusion, difficulty concentrating, difficulty speaking, dizziness, drowsiness, fast heartbeat, headache, hunger, nausea, nervousness, numbness or tingling of the lips, fingers, or tongue, sweating, tiredness, trembling, weakness.
Many of these side effects can be managed, and some may go away on their own over time. Although most of the side effects listed above don’t happen very often, they could lead to severe problems if you do not seek medical attention.
Stop taking the medication and seek immediate medical attention if any of the following occur: rash or blisters all over the body, seizures, symptoms of Anaphylaxis (severe allergic reaction) (e.g., Hives, difficulty breathing, wheezing, fast heart rate, sweating, swelling of the face and throat) and unconsciousness.
Some people may experience side effects other than those listed. Check with your doctor if you notice any symptoms that worry you while taking Apidra Solostar (Insulin-glulisine-rapid).
The metabolism of glucose can be affected by a number of drugs and insulin doses should be adjusted accordingly.
Some medications, such as Apidra, may cause hypoglycemia by increasing the blood-glucose-lowering effects of insulins, such as oral antidiabetic medications, pramlintide, ACE inhibitors, disopyramide, fibrates, fluoxetine, monoamine oxidase inhibitors, propoxyphene, pentoxifylline, salicylates, somatostatin analogs, and sulfonamide antibiotics.
The number of drugs that could reduce Apidra’s blood-glucose-lowering effect is a long list. These include corticosteroids, niacin, danazol, diuretics, sympathomimetic agents (e.g., epinephrine, albuterol, terbutaline), glucagon, isoniazid, phenothiazine derivatives, somatropin, thyroid hormones, estrogens.
Insulin’s blood-glucose-lowering effect may be affected by beta-blockers, clonidine, lithium salts, and alcohol.
Hypoglycemia and hyperglycemia can sometimes occur after pentamidine use.
In patients taking beta-blockers, clonidine, guanethidine, or reserpine, signs of hypoglycemia may be reduced or absent.
Since this medication contains insulin glulisine, it needs to be consumed within 15 minutes of having a meal. You may not be on a regular eating or dosing schedule. If you miss a dose, do not use two at the same time.
When given intravenously, excess insulin can lead to hypoglycemia and hypokalemia. Hypoglycemia that is mild can usually be treated with oral glucose. The dose of your medications, your diet, or your exercise regimen may need to change. Glucagon or concentrated intravenous glucose may be administered in cases of more severe hypoglycemia associated with coma, seizure, or neurologic impairment. Because hypoglycemia may recur after apparent clinical recovery, sustained carbohydrate intake and observation may be required. Hyperkalemia must be corrected appropriately.
Contact the Poison Help Line at 1-800-222-1222 or seek medical attention immediately as it can lead to life-threatening hypoglycemia. You should seek immediate medical attention if you experience symptoms such as drowsiness, confusion, numbness, tingling in your mouth, difficulty speaking, blurred vision, muscle weakness, seizures, or loss of consciousness.
There are no well-controlled clinical studies of the use of Apidra in pregnant women. In view of the fact that animal reproduction studies are not always predictive of human responses, this drug should only be used during pregnancy if the benefit outweighs the risk to the fetus. The maintenance of good metabolic control during and before pregnancy is essential for patients with diabetes or a history of gestational diabetes. Insulin requirements may decrease during the first trimester, generally increase during the second and third trimesters, and rapidly decline after delivery. Careful monitoring of glucose control is essential in these patients.
Taking the Apidra SoloStar pen while pregnant (or planning to become pregnant) is not recommended.
There are no well-controlled clinical studies of the use of Apidra in pregnant women. In view of the fact that animal reproduction studies are not always predictive of human responses, this drug should only be used during pregnancy if the benefit outweighs the risk to the fetus. The maintenance of good metabolic control during and before pregnancy is essential for patients with diabetes or a history of gestational diabetes. Insulin requirements may decrease during the first trimester, generally increase during the second and third trimesters, and rapidly decline after delivery. Careful monitoring of glucose control is essential in these patients.
Taking the Apidra SoloStar pen while pregnant (or planning to become pregnant) is not recommended.
SoloStar Pens should be kept out of reach and sight of children.
Store your SoloStar in a cool location (2°C – 8°C) until first use. Avoid freezing your SoloStar Pen. It should not be placed near the freezer compartment of your refrigerator or next to a freezer pack. When you are ready to inject your SoloStar, take it out of cool storage one to two hours before injecting. Insulin that is cold is painful to inject.
As soon as you take your SoloStar Pen out of cool storage, either for use or as a spare, you can use it for up to 28 days. If possible, keep it at room temperature (15 – 25°C) and do not store it in the refrigerator. Once the 28-day shelf life has expired, any remaining insulin should be thrown away.
SoloStar should not be used after the expiration date printed on the label or if the ink is cloudy, colored, or if you see particles. Keep SoloStar away from light. If your local authorities require it, dispose of your used SoloStar.
| Quantity | 5 Pens, 10 Pens, 15 Pens, 20 Pens, 25 Pens |
|---|